CHEMISTRY
STPM 962/2
1. When one mole of sodium bromide dissolves in water,
the enthalpy change is -8 kJ mol-1. If the lattice energy of sodium
bromide and the hydration energy of the Na+ ion are -736 kJ mol-1
and -406 mol-1 respectively, what is the hydration energy of the Br-
ion?
A -1150
kJ mol-1 B -338 kJ mol-1 C -322
kJ mol-1 D +1 150 kJ mol-1
2. Which of the following is not required in the calculation of the lattice
energy of calcium oxide sing Born Haber cycle ?
A. Enthalpy of
hydration B.
Enthalpy of ionization C. Enthalpy
of atomization D. Electron
affinity
3. The
standard enthalpy of formation of ethanol is -278 kJ per mole. Which equation
relates to the formation of ethanol under standard conditions?
A.
2C(g) + 3H2(g) + 1/2O2 (g) à C2H5OH(l)
B.
2C(s) + 3H2(g) + 1/2O2 (g) à C2H5OH(l)
C.
2C(g) + 3H2(g) + 1/2O2 (g) à C2H5OH(l)
D.
4C(g) + 6H2(g) + O2 (g) à 2C2H5OH(l)
4. Which of the following is not a
disproportionation reaction ?
A.
Cl2 + NaOH à NaOCl + NaCl +H2O
B.
3Cl2 + 6NaOH à NaClO3 + 5NaCl + 3H2O
C.
4HCl + MnO2 à Cl2 + MnCl2 + 2H20
D.
3NaOCl à NaClO3 + 3 NaCl
5. The
relationship between electrode potential and ion concentration is given by the
following Nernst equation.
For the reaction Co(s) + Ni2+ (aq)à Co2+(aq) + Ni(s), the E value is
+0.0300 V. If the concentration of Co2+
is reduced to 0.1000 mol dm-3 and the concentration of Ni2+
is maintained, what is the value of Eq for the cell under the same
conditions?
A-0.0292 V B+0.0004
V C +0.0596
V D
+0.0892 V
6. The graphs below show the variation in three physical properties of
elements in Period 3 of the Periodic Table
What are the physical properties that the graphs refer
to?
I II III
A Boiling point Conductivity Vaporisation enthalpy
B Melting point Atomic
radius First ionization energy
C Melting point Conductivity First ionization energy
D Conductivity Atomic
radius First ionization energy
7. . The successive
ionization energies, in kJ mol-1 , of an element in the Periodic
Table are given below.
940 (First), 2080, 3090, 4140, 7030, 7870, 16 000, 19
500
In which group in the Periodic Table is this element
likely to be located?
A Group 13 B
Group 14 C Group 15 D Group 16
8. The carbonates of the Group 2
elements (beryllium to barium) decompose according to the equation
MCO3(s)→MO(s)
+ CO2(g).
Which of the following combinations is correct when
descending Group 2?
Lattice energy of MCO3 Lattice energy of MO Decomposition temperature of MCO3
A Increases Increases Increases
B Increases Decreases
Increases
C Decreases Increases
Increases
D Decreases Decreases
Increases
9. Which statement best explains why the thermal stability of the
carbonates of Group 2 metals of the Periodic Table increases going down the
group?
A The polarization power of the metal ion towards the
CO32- ion decreases
B The strength of ionic bond in the metal carbonate
increases
C The radius of the metal ion increases.
D The electronegativity of the metal decreases
10.
Which tetrachloride can bleach the colour of litmus
paper at room temperature?
A SiCl4 B GeCl4 C SnCl4
D D
PbCl4
11.
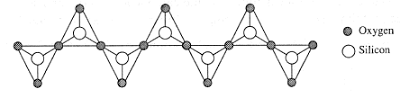
The diagram below shows the structure of a type of
silicate.
Which of the following statements is correct with
regard to the above structure?
A It
represents the structure of asbestos.
B The
repeating unit is SiO44-
C The
structure is found in the mineral pyroxene.
D The ratio
of Si to O is 1 : 4.
12. The reactivity of the halogens
Cl2, Br2 and I2 decreases down Group 17 of the
Periodic Table. Which property does not influence the trend in the reactivity
of the halogens?
A Atomic size B
Electron affinity C
Ionization energy D Bond energy
13.
Most of the
transition elements and their compounds are heterogeneous catalysts which are
useful for various reactions. The transition elements possess this catalytic
power because they ……
A can form complexes
B can act as reducing agents
C can alter their oxidation number
D have a good adsorption characteristic
14.
The colour of a
transition metal complex ion is determined by its ligand and geometry.
Which species has a hexadentate ligand?
A Na2[CuCl4] B [Ag(NH3)2]+ C [Ni(EDTA)]2- D [Co(C204)3]3-
15.
The solution produced when chlorine is bubbled through
dilute sodium hydroxide can be used
A as pesticide B
as bleaching agent C
in sterilizing water D to extract bromine
Answer:
1.
B 2. A 3.
B 4. C
5. C 6. B 7. D
8. D 9. B 10. D
11.C 12.C 13. D
14. C 15. B
GOOD LUCK.......
Part A
Time
: 30 minutes
Name:
_____________________________ Marks
: ___________________________
1.
In the construction of cardiac pacemakers, it is possible to use a very small
magnesium electrode which creates an electrical cell with the inhaled oxygen.
The relevant half-cells are as follows.
Mg2+ + 2e
→ Mg E0 = -2.38V
½
O2 + 2H+ + 2e → H2O E0 = +1.23V
Under
standard conditions, the cell e.m.f would be 3.61 V, but in the body a
potential of 3.25 V is more usual.
What
is the best explanation of this lower e.m.f ?
A
The small size of the magnesium electrode
B
The low concentration of Mg2+ ions surrounding the magnesium
electrode
C
The high resistance of the body fluids surrounding the electrodes.
D The pH between 7 and 8 of the body fluid surrounding the
electrodes.
2.
Which value would be required to estimate the lattice energy for the
hypothetical ionic compound, MgH2?
A The electron affinity of hydrogen
B
The first ionization energy of hydrogen
C
The magnesium-hydrogen bond energy
D
The standard enthalpy change of formation of MgH2
3.
Consider the sequence of oxides given below. Which series shows an increasing
order of melting point?
A
Cl2O7, Al2O3, SO3, SiO2 B SO3, Cl2O7,
Al2O3, SiO2
C SO3, Cl2O7, SiO2,
Al2O3 D SiO2,
Al2O3, Cl2O7, SO3
4.
H2(g) → 2H(g) ∆H = +436KJ
Br2(g) → 2Br(g) ∆H = +194KJ
H2(g) +Br2(g) → 2HBr(g)
∆H = -104KJ
Use the above
data to determine the ∆H for the following reaction.
H (g) + Br(g) → HBr(g)
A
-288 kJ B -367 kJ C
+263 kJ D
+526 kJ
5.
The following oxides are amphoteric except
A.
Al2O3 B.
PbO2 C.
SnO D. SiO2,
6.
Astatine is an element in Group 17 of the Periodic Table. What are the expected
properties of astatine at 250 C?
Physical state
|
Oxidizing power
|
Color
|
|
A
|
Solid
|
Weak
|
Black
|
B
|
Solid
|
Strong
|
Black
|
C
|
Solid
|
Strong
|
Purple
|
D
|
Liquid
|
Weak
|
Purple
|
7.
Which of the following statements is
true of glass?
A.
It consists of well-arranged silicate units B. It
withstands the action of strong alkaline.
C. It softens at a wide range of
temperatures. D. It can be coloured
by adding soda lime
8.
Compared to barium, beryllium
A. is a stronger reducing agent B. forms a more basic oxide.
C. reacts more vigorously with water. D. form more covalent compounds
9. Which of me following block--d elements does not
show general characteristics of a transition element?
A. Scandium B. Vanadium C. Copper D. Nickel
10. A steady current flows through an aqueous
solution of potassium iodide for 4825 seconds. The iodine produced requires
12.5 cm3 of 0.80 mol dm-3 sodium thiosulphate(VI) for complete
reaction. What is the value of the current flowing in this electrolytic cell? [
I2 ≡ 2S2O3 2- ; 1F = 96, 500 C mol-1]
A 0.1
A B 0.2
A C 0.3 A D 0.4 A
11.
Silicon (IV) oxide is a solid whereas carbon (IV) oxide is a gas. This is
because
A carbon
is more electronegative than silicon
B the
silicon-oxygen bond is stronger than the carbon-oxygen bond
C silicon(IV)
oxide partially ionic whereas carbon(IV) oxide is purely covalent
D silicon (IV) oxide is
a giant molecule whereas carbon (IV) oxide is a simple molecule
12.
Chlorine gas is bubbled through aqueous sodium hydroxide. What is the use of
the solution obtained?
A Deodorant B Antiseptic C Bleaching
agent D Aerosol propellant
13.
The oxides of group 14 which are neutral, acidic and amphoteric are;
Acidic
|
Neutral
|
Amphoteric
|
|
A
|
GeO
|
PbO
|
PbO2
|
B
|
SiO2
|
CO
|
SnO
|
C
|
CO
|
SiO2
|
GeO2
|
D
|
CO2
|
CO
|
SnO2
|
14.
X, Y and Z are elements in the same short period of the Periodic Table. X
reacts with both dilute hydrochloric acid and aqueous sodium hydroxide to form
hydrogen. The oxide of Y is basic and the oxide of Z is acidic.
What
is the order of increasing proton number for these elements?
A
X, Y, Z B
X, Z, Y C Y, X, Z D Z, X, Y
15.
Which graph correctly describes a trend found in the halogen group?






Thanks a lot! =)
ReplyDeleteCasinos that work in Vegas, Nevada - Dr.MCD
ReplyDeleteThe casinos in Las 서귀포 출장마사지 Vegas, Nevada are run by the Wynn Resorts Limited 제주 출장샵 and run by 거제 출장마사지 the Nevada Gaming Control Board. Las Vegas 고양 출장마사지 has been the center of 태백 출장마사지 the Las Vegas